Complete your
view of COVID-19
with T cell testing.
Use T-SPOT.COVID to reliably assess
the T cell response to COVID-19 vaccination
and SARS-CoV-2 infection
The T-SPOT.COVID test antigen mix optimized for SARS-CoV-21
The T-SPOT.COVID test is a standardised ELISPOT based technique for qualitative detection of a cell mediated (T cell) immune response to SARS-CoV-2 in human whole blood, intended for use as an aid in identifying and monitoring individuals with a T cell immune response to COVID-19 vaccination or SARS-CoV-2 infection. The test uses the established T-SPOT Technology with an optimized antigen mix, based on SARS-CoV-2 structural proteins, spike (S) and nucleocapsid (N), and allows the maximum breadth of the immune response to be measured.
The T-SPOT.COVID test1
CE marked for IVD use
- Used to detect / monitor a T cell response to both COVID-19 vaccination and SARS-CoV-2 infection
- Measures SARS-CoV-2 specific S + N protein responses separately
- Proven to detect evidence of a T cell-mediated immune response to SARS-CoV-2 infection in PCR positive patients with negative serology test results
Update to package insert
The package insert has been updated to reflect additional published data supporting the use of the T-SPOT.COVID test for vaccine monitoring. View more details about the changes by clicking here.
Why partner with Oxford Immunotec?
A leading global diagnostics company with a nearly 20-year history of transforming T cell science into meaningful insights using the T-SPOT Technology. The T-SPOT Technology is proven in TB diagnostics with > 20 million tests shipped to > 50 countries where the T-SPOT.TB test is approved for clinical use.
_Why offer COVID-19 T cell testing?_
Performance of the T-SPOT.COVID test
| Time after positive PCR results | T-SPOT.COVID positive agreement | T-SPOT.COVID negative agreement |
| < 60 days | 96.6% (84/87) | – |
| > 60 days | 83.3% (40/48) | – |
| N/A | – | 98.0% (96/98) |
- Positive agreement: 135 individuals with a previous positive COVID-19 test result
- Negative agreement: 98 individuals at low risk of acquiring infection and who have never had a positive COVID-19 test result
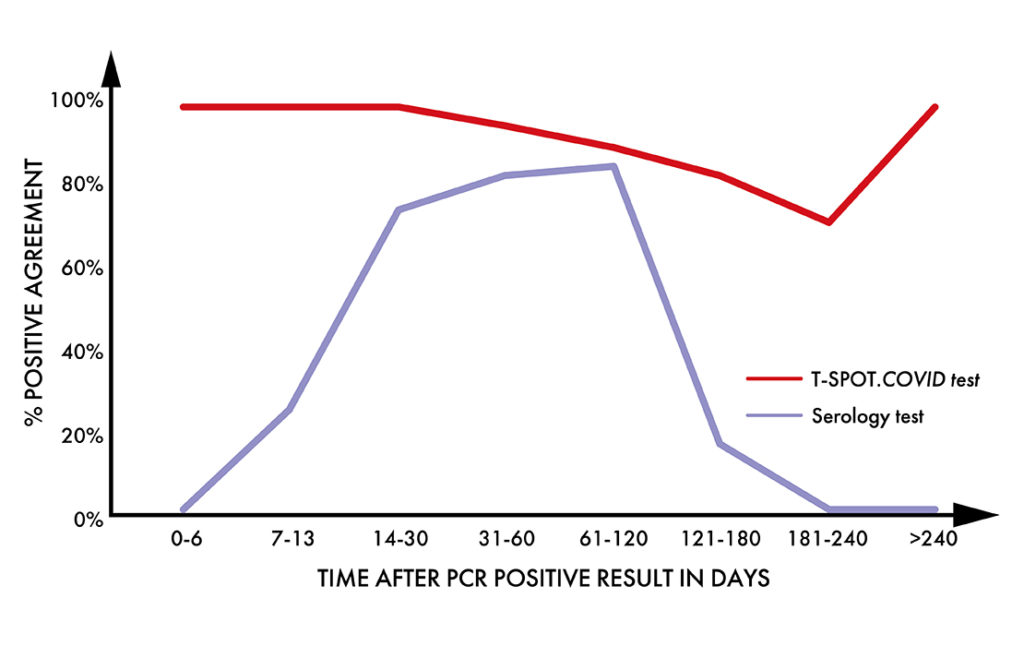
Higher positive agreement than a leading serology test1
In a study of 135 individuals, with a previous positive PCR result for SARS-CoV-2 infection, samples were analysed by both the T-SPOT.COVID test and a leading serology test.
- T cell positive agreement remained high throughout the length of the study
- Serology positive agreement decreased over time
- Serology positive agreement was low before 14 days
Serology alone is not enough1
Research shows T cell response and antibody immune response are independently correlated to protection against SARS-CoV-2. Antibody response represents half of the immune system and T cells are the other half.
Further analysis of the study cohort evaluated overlap between the two test methods.
The T-SPOT.COVID test identified 45% more PCR positive individuals than serology testing alone

_FAQ_
- How does the T-SPOT technology work?
Our T-SPOT.COVID test based on ELISPOT technology is normalised for both cell number and culture conditions. This means that the test standardises the number of cells and removes serum factors that could adversely affect results, making it the most sensitive and specific test for T cell measurement. A blood sample is collected using routine phlebotomy and a standard blood collection tube from which a subset of white blood cells, known as peripheral blood mononuclear cells (PBMCs), are isolated. The cells are washed, counted and normalised to create a standard cell suspension. A standard number of cells are added into specially designed plates and stimulated with antigens specific to the disease under study. Cells responding to these antigens release a chemical messenger known as a cytokine. Cytokine antibodies are used to directly capture the cytokine as it is released by the cells. A secondary labelled antibody is added and binds to the captured cytokine. A detection reagent is added and reacts with the secondary labelled antibody. This reaction produces spots, which are a footprint of where the cytokine was released. Spots are then enumerated. - What does a positive T-SPOT.COVID test result mean?
A positive test result means that the patient has T cells that are reactive to the SARS-CoV-2 specific peptides used in the T-SPOT.COVID test. It is highly likely that they have been exposed to the SARS-CoV-2 virus. - What does a negative test result mean?
A negative test result means that the patient does not have T cells that are reactive to the SARS-CoV-2 specific peptides used in the test. It is therefore unlikely that they have been exposed to the SARS-CoV-2 virus. - What regulatory approval does the T-SPOT.COVID test have?
The T-SPOT.COVID test is CE marked and has been submitted to the FDA for Emergency use authorisation.
T cells in COVID-19
Helen Fletcher, Professor of Immunology at the London School of Hygiene and Tropical Medicine, talks about the importance of T cells in COVID-19 and answers some topical questions, including:
- What role might T cells have in COVID-19?
- Are antibodies or T cells more important to COVID-19?
- How might T cells be used for testing?
Public Health England2
A prospective cohort study performed by Public Health England used the T-SPOT research use only platform to provide detailed information on SARS-CoV-2-specific T cells in immune responses to SARS-CoV-2 infection. In this study, 2,826 individuals identified as keyworkers were tested for anti-spike IgG and SARS-CoV-2 responsive T cells.
Key findings
- T cell responses could be detected in individuals who had tested positive for SARS-CoV-2 using PCR, but were seronegative
- T cells may play a role in protection against re-infection with the SARS-CoV-2 virus, even in the absence of detectible antibody

Resource Centre
REFERENCES
- T-SPOT.COVID Package Insert: T-SPOT.COVID-PI-UK-0001
- Wyllie D, Mulchandani R, Jones HE et al. SARS-CoV-2 Reactive T cell numbers are associated with protection from COVID-19: A prospective cohort study. medRxiv. doi: 10.11012020.11.02.20222778